March 20, 2019

March 20, 2019


Operating a business under a Consent Decree is extremely difficult; there is no area of the business that goes unaffected. In addition to fines that could reach $500 million, a three to five-year Consent Decree can result in additional penalties, remediation expenses, lost sales, and diminished stock value.
That’s just the financial aspect. A Consent Decree can also damage a company’s image and culture. The impact also extends to customers and patients who now have doubts about the effectiveness and safety of the company’s products.
It requires a well-planned effort to adequately address the terms of a Consent Decree while managing the company’s day-to-day affairs over the long term. This effort goes far beyond developing and executing a remediation Project Plan. It must include tactics that allow the company to survive the Consent Decree and emerge from the remediation efforts prepared to thrive. It is the epitome of changing the flat while continuing to pedal.
A Consent Decree is a legal agreement between a company and the US Government that is enforced by the U.S. Department of Justice after court approval. It details the voluntary actions pledged by the company to remedy nonconformances, so the company can avoid litigation. In exchange for agreeing to the terms of the Consent Decree, a company may be allowed to continue manufacturing and selling products under a heightened level of scrutiny. In some situations, FDA may decide that production should be halted until certain remediation efforts are completed.
Consent Decrees are usually imposed due to continual nonconformance to current good manufacturing practices (GMPs). Continual nonconformance is defined as recurring Form 483 observations transmitted to a company over the course of several audits and inspections. The FDA uses Consent Decrees as a tool to improve a company’s culture with regards to compliance by obtaining a commitment to performing corrective actions in a timely manner.
Leaders must be made aware that they are no longer simply managing a business. They are managing a business within a Consent Decree environment. This means they must take a holistic approach that coordinates remediation with ongoing business operations. Each decision must be compliance-driven while also focusing on the life of the business once the Consent Decree ends. This level of managerial effort must be sustained year after year.
The culmination of this effort is the creation of a culture of compliance – a culture that respects and demands compliance from all functions within the business. Not only must this new culture be defined for every role in every function, the new expectations it brings must be communicated to employees and properly reinforced. Individuals and functions that do not live up to the new standards must be held accountable so that employees at all levels understand the importance of compliance and embrace compliant behaviors as core elements of their work culture.
Consent Decrees are in place for so long because remediation takes time. The Consent Decree’s Project Plan is the roadmap for this process, which is why it is critical that you have a well-thought-out Project Plan that lays out precisely what the deliverables are, when they are to be delivered, and by whom. It’s this plan that lets everyone involved understand what their role is within the project.
Precision is one hallmark of a good Project Plan. Realism is another. Remember that a Consent Decree is a negotiated agreement wherein the company tells the FDA what the company will do, and when it will do it. There are consequences to not completing deliverables on time, and penalties can be as high as $10,000 per day per deliverable missed. As such, it’s vitally important that care be taken to create a Project Plan with deliverables that the company can reasonably meet while operating under stressful circumstances.
Surviving a Consent Decree requires a comprehensive and integrated approach to managing all aspects of the business along with remediation efforts. For instance, the company must strike a fine balance between manufacturing their product and bringing processes within cGMP guidelines. The Project Plan says that the company must re-validate certain systems, but, the product still has to be manufactured and sold. This places stress on the system because the time it takes to re-validate systems takes away from production time, making communication and good project planning critical components in order to succeed.
Effectively operating under a Consent Decree means having an unwavering focus on accountable governance. One way to accomplish this is to have a group of staff members specifically focused on managing the business under the Consent Decree. This team should be made up of staff from every area of the company as well as outside experts. The primary functions of this team are to interact with FDA and its representatives and manage the project plan to ensure that deliverables are being met on time. It is their job to balance the potential conflicting interests between functions relating to basic operations and components of the Consent Decree.
It’s crucial that companies do all that they can to prevent a major regulatory action like a Consent Decree from being initiated. This includes seeking assistance when early warning signs arise.
To stay in compliance, count on ProPharma Group to provide you with an independent, third-party perspective on your organization’s regulatory compliance. Let us examine your quality systems to look for issues that could lead to an observation of noncompliance from FDA. After identifying any issues, we will provide recommendations for correcting them, and if needed, we will help you implement these recommendations.
Surviving a multi-year Consent Decree is doable. However, it’s always best to avoid that level or remediation by staying compliant. Contact us today to learn more about our experience helping companies overcome and/or avoid regulatory action and how we can help you do the same thing.
TAGS: Regulatory Sciences
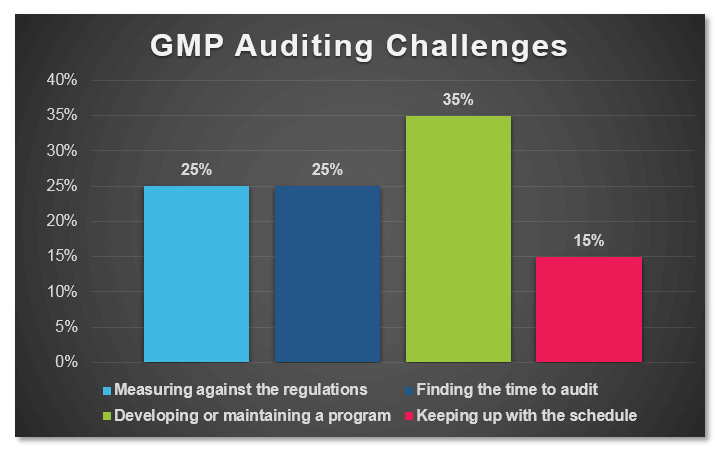
September 5, 2018
In a recent poll conducted by ProPharma Group, the question “What is your biggest GMP auditing challenge?” was posed to Quality professionals in the drug manufacturing industry. The following graph...
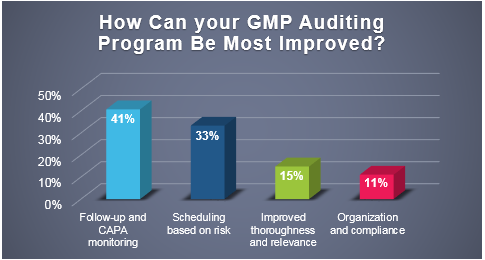
November 29, 2018
Recently, ProPharma conducted a poll to quality professionals across the country to understand the challenges that FDA regulated companies face in managing their GMP auditing programs. As depicted in...